Te digestive system consits of approximately a dozen organs that work cooperatively to digest (both mechanically and chemically) macromolecules and absorb their building blocks. The next seven posts examine these organs in isoltaion to help us appretiate the complexity of digestion which we most often take for granted.
Wednesday, February 16, 2011
Tuesday, February 15, 2011
The Mouth, Pharynx, and Esophagus
Digestion has two main process, mechanical and chemical:
The Pharynx
- mechanical: Breaking down of food into smaller pieces by muscle contractions, chewing, and smashing, so that food can be absorbed by cells
- Chemical: breaking down food into smaller molecules (simple molecules) by chemical reactions, such as enzymes, readying it for absorption

- The tongue: sensor receptors called tasted buds (on the tongue are activated when food is present and the nerve impulses travel by the way of cranial to the brain that is why the food tastes good)
- The teeth chew the food into smaller molecules to increase the surface area so that the Enzymes can process them faster
- Tonsil: protects the body from infections (in the mucus membrane)
- Salivary Glands: There are three salivary glands, they produce enzymes (salivary amylase) which begin the process of digesting starch (making it maltose)

The Pharynx
- Receives air from nasal cavity (nose) -and food from oral cavity (the mouth)
- The food passage is called the esophagus and the air passage trachea.
- During swallowing the soft palates move to the back to close off the nasal cavity (rooftop between the oral cavity and the nasal cavity); while the trachea moves under the epiglottis to cover the glottis so that we do not breath while swallowing.
- Is a muscular tube that passes from the pharynx eventually joining with the stomach
- Is normally collapsed, but it opens when we are swallowing
- peristalsis is a rhythmic contraction that pushes the food along the digestive tract, though this can happen even with no food which creates a lump in the throat sensation
- the esophagus is just a passageway though and has no role in chemical digestion
- sphincters are valves that prevent acidic content of the stomach from coming up into the esophagus which creates heartburn
By: Rio, Mahtab, Maede, Desiree
The stomach
-The stomach is a J-shaped organ that is on the left side of the body

-It is the continuation of the esophagus above and the duodenum of the small intestine below
-The stomach stores food and aids in digestion
-The wall of the stomach has deep folds which disappears as the stomach fills
-stomach has a capacity of approx. 1liter
-the wall churns mixing the food with the gastric juices (gastric always refers to the stomach)
-the rugae make it easier to churn the foodàcreates a rougher surface for the food to be mixed
-The columnar epithelial lining of the stomach mucosa has millions of gastric pitsàwhich lead into gastric glands the gastric glands produce gastric juices
-gastric juices produce an enzyme called pepsin, which digests protein and HCl and mucus
-the enzyme pepsin breaks the protein into peptides protein+wateràpeptides
-the HCl causes the stomach to have a low pH of about2 this is beneficial because it kills most bacteria present in food
-HCl does not digest food, it breaks down the connective tissue of meat and activates pepsin
-The wall of the stomach is protected by a thick layer of mucus secreted by goblet cells in its lining
-if HCl penetrates the mucus the HCl can break down the wall and an ulcer will occur
-Alcohol is absorbed in the stomach but food substances are not
-normally the stomach empties in about 2-6 hours

-when food leaves finally it is a thick soupy liquid called chyme
-chyme enters the small intestine through the way of the Pyloric sphincter the link between the esophagus and the stomach.
-The function of the Cardiac sphincter is to prevent the gastric acids (Hydrochloric acid) from entering the esophagus also known as heart burn. This sphincters muscles contract to open and close as food travels down the esophagus.
-Pyloric Sphincter: the function of this sphincter is to regulate the flow of chyme into the duodenum and it prevents the regurgitation of chyme from the small intestine to the stomach, chyme enters the small intestine through squirts by the sphincter because it repeatedly opens and close
Liver & Gall bladder
Definition of the liver: It's the largest gland in our body which lies mainly in the upper right section of the abdominal cavity, under the diaphragm.
Structure: the liver has two main lobes, the right lobe & the smaller left lobe. It contains approximately 100,000 lobules that serve as its structural & functional units.

Liver's functions in the body:
-as the blood from the hepatic portal vein passes through the liver, it removes poisonous substances & detoxifies them.
-the liver also removes nutrients & works to keep the contents of the blood constant.
-it removes and stores iron & the fat soluble vitamins A,D,E,K, & B12.
-the liver makes the plasma proteins from amino acids.
-helps regulate the quantity of cholesterol in blood.
ex. the conversion of amino acids to glucose necessitates deamination, the removal of amino groups to form urea.

Liver's functions in the digestive system:
-maintains the blood glucose level at about 0.1% even though a person eats intermitently.
-when insulin is present, any excess glucose present in blood is removed & stored by the liver as glycogen.
-between meals, glycogen is broken down to glucose, which enters the hepatic veins & in this way, the blood glucose level remains constant.
-if the supply of glycogen is depleted, the liver converts glycogen ( from fats) and amino acids to glucose molecules.
-the liver produces bile, which is stored in the gall bladder.
Bile's definition:
-Bile is a yellow-green fluid that is made by the liver, stored in the gallbladder and passes through the common bile duct into the duodenum where it helps digest fat.
-The principal components of bile are cholesterol, bile salts, and the pigment bilirubin.
why does bile have a green colour?
bile has a yellowish-green colour because it contains the bile pigment bilirubin, derived from the breakdown of hemoglobin, the red pigment of red blood cells.
Definition of gall bladder:
-is a pear shaped, muscular sac attached to the surface of the liver.
-about 1000 ml of bile are produced by the liver each day, any excess is stored in the gall bladder.
-water is re-abosorbed by the gall bladder so that bile becomes a thick, mucus like material.
-when needed, bile leaves the gall bladder and proceeds to the duodenum via the common bile duct.
-cholesterol content of bile can come out of solution and forms crystals.
-if the crystal grows in size, they form gall stones.
By: Rojin, Nicole, Sarah, Fred
Structure: the liver has two main lobes, the right lobe & the smaller left lobe. It contains approximately 100,000 lobules that serve as its structural & functional units.

Liver's functions in the body:
-as the blood from the hepatic portal vein passes through the liver, it removes poisonous substances & detoxifies them.
-the liver also removes nutrients & works to keep the contents of the blood constant.
-it removes and stores iron & the fat soluble vitamins A,D,E,K, & B12.
-the liver makes the plasma proteins from amino acids.
-helps regulate the quantity of cholesterol in blood.
ex. the conversion of amino acids to glucose necessitates deamination, the removal of amino groups to form urea.

Liver's functions in the digestive system:
-maintains the blood glucose level at about 0.1% even though a person eats intermitently.
-when insulin is present, any excess glucose present in blood is removed & stored by the liver as glycogen.
-between meals, glycogen is broken down to glucose, which enters the hepatic veins & in this way, the blood glucose level remains constant.
-if the supply of glycogen is depleted, the liver converts glycogen ( from fats) and amino acids to glucose molecules.
-the liver produces bile, which is stored in the gall bladder.
Bile's definition:
-Bile is a yellow-green fluid that is made by the liver, stored in the gallbladder and passes through the common bile duct into the duodenum where it helps digest fat.
-The principal components of bile are cholesterol, bile salts, and the pigment bilirubin.
why does bile have a green colour?
bile has a yellowish-green colour because it contains the bile pigment bilirubin, derived from the breakdown of hemoglobin, the red pigment of red blood cells.
Definition of gall bladder:
-is a pear shaped, muscular sac attached to the surface of the liver.
-about 1000 ml of bile are produced by the liver each day, any excess is stored in the gall bladder.
-water is re-abosorbed by the gall bladder so that bile becomes a thick, mucus like material.
-when needed, bile leaves the gall bladder and proceeds to the duodenum via the common bile duct.
-cholesterol content of bile can come out of solution and forms crystals.
-if the crystal grows in size, they form gall stones.
By: Rojin, Nicole, Sarah, Fred
The Pancreas!
- It is an elongated and somewhat flattened organ that has both an endocrine and exocrine function.
- As an endocrine gland, it secretes insulin and glucagon, hormones that help keep the blood glucose level within normal limits.
- Most Pancreatic cells produce pancreatic juice, which contains Sodium Bicarbonate and digestive enzymes for all types of foods.
- Pancreatic amylase digests starch, trypsin digests protein, and cipase digests fat.
The two antagonistic hormones insulin and glucagon, both produced by the Pancreas, maintain the normal level of glucose in the blood.
Insulin:
- As an endocrine gland, it secretes insulin and glucagon, hormones that help keep the blood glucose level within normal limits.
- Most Pancreatic cells produce pancreatic juice, which contains Sodium Bicarbonate and digestive enzymes for all types of foods.
- Pancreatic amylase digests starch, trypsin digests protein, and cipase digests fat.
The two antagonistic hormones insulin and glucagon, both produced by the Pancreas, maintain the normal level of glucose in the blood.
Insulin:
-Is secreted when the blood glucose level is high, which usually occurs just after eating.
-Insulin stimulates how much glucose is taken by cells
-In liver and muscle cells glucose is stored as glycogen
-In muscle cells the breakdown of glucose supplies energy for preotein metabolism
-In fat cells glucose break down supplies glycerol for the formation of fat
Glucagon:
Glucagon:
- Is secreted from the Pancreas, and this happens usually between meals when the blood glucose level is low.
-Glucogon stimulates the liver to break down glycogen to glucose to use fat and protein in preference to glucose as an energy source
Diabetes is a common hormonal disease where liver cells and all body cells are unable to take up and or metabolize glucose.

Diabetes is a common hormonal disease where liver cells and all body cells are unable to take up and or metabolize glucose.

There are two different types of diabetes,
Type 1, Insulin dependent, where the pancreas is not producing insulin
- This requires the individual to acquire daily insulin injections, if a diabetic misses an insulin shot or a meal they can become anxious or have perspiration, pale skin & shallow breathing
- Sugar or juice can quickly contract hypoglycemia
Type 2, non-insulin dependant diabetes
- In obesity or inactive
- Pancreas produces insulin but liver and muscle cells do not respond in a usual manner
- Low fat, low sugar diet is needed
Type 1, Insulin dependent, where the pancreas is not producing insulin
- This requires the individual to acquire daily insulin injections, if a diabetic misses an insulin shot or a meal they can become anxious or have perspiration, pale skin & shallow breathing
- Sugar or juice can quickly contract hypoglycemia
Type 2, non-insulin dependant diabetes
- In obesity or inactive
- Pancreas produces insulin but liver and muscle cells do not respond in a usual manner
- Low fat, low sugar diet is needed
An Excellent Video!! http://www.youtube.com/watch?v=1l2GTGEwZOY&playnext=1&list=PL5DB15064FCD04401
Small Intestine
The small intestine comes between the gastrointestinal tract and the large intestine. It has a small diameter and is 6 meters long. Its surface area is approximately the same as that of a tennis court. The small intestine is slightly basic because pancreatic juice contains sodium bicarbonate(NaHCO3).

A villus contains blood capillaries and a small lymphatic capillary called a lacteal. Sugars and amino acids enter the blood capillaries. Glycerol and fatty acids enter the epithelial cells. In the cells they are joined and packaged as lipoprotien droplets and then enter the lacteal. After this absorption process finishes, the nutrients are transported to the organs of the body through the blood stream.


By: Katie, Monica, Anne, and Anna

The surface area of the small intestine contains finger-like projections called villi. They have a soft and velvety appearance. Each villus has thousands of microscopic extensions called micro-villi. They give villi a fuzzy border known as a "brush border". This border increases the surface area and helps in the absorption of nutrients. The nutrients are absorbed into the vessels of a villus.


By: Katie, Monica, Anne, and Anna
Duodenum
Duodenum
· This is the first 25 cm of the small intestine
· Where ducts from liver and pancreas join info into the duodenum in a single duct
· Receives bile from liver, and pancreatic juice; bile is produced in the liver.
Bile
· This is how we emulsify fat
· Emulsification- cause large fat droplets to disperse in water
· Bile s a mechanical digestive process
· This is mechanical because bile physically rips the fat apart
· Bile aids the digestion and absorb the fats
The Duodenum Recieves Pancreatic Juice
· Pancreatic juice contains sodium bicarbonate (NaHCo3), which neutralizes chime. Thick semi liquid, acidic, food material that passes from the stomach to the small intestine and it has a slightly basic pH around 8
· First job: juice helps protect the rest of the small intestine from the acidity of chime
· Pancreatic amylase digests starch into maltose
· Tryspin breaks down proteins into peptides
· Lipase digests fat into fatty acids and gycerol
· Nuclease breaks down RNA + DNA into Nucleatide
Small intestine
· Maltase [Maltose + H2O à Glucose + Glucose]
· Peptidases [Peptide + H2O à Amino Acids]
· Nucleotidases [Nucleotide + H2O à Base + Sugar + Phosphate]
Enzymes and Hormones
· The duodenum is responsible for hormones that trigger the pancreatic duct to open pancreatic juice and bile
Large Intestine
Struction and Functions

Parts of the large intestines are:

Cecum – the first part of the large intestine

- Starts in the right lilac region of the pelvis, just at or below the right waist, where it is joined to the bottom end of the small intestine. From here it continues up the abdomen, then across the width of the abdominal cavity, and then it turns down, continuing to its endpoint at the anus.
- It is about 1.5m long.
- It takes about 16 hours to finish up the remaining processes of the digestive system.
- Absorbs water, salt and some vitamin.
- Storage of indigestible remains.
- Consists of cecum and colon.
Parts of the large intestines are:

Cecum – the first part of the large intestine
- Taeniae Coli – three bands of smooth muscle
- Haustra – bulges caused by contraction of taeniae coli
- Epiploic Appendages– small fat accumulations on the viscera
- The ascending colon
- The transverse colon
- The descending colon
* Flora means the sum of bacteria
- Large intestine has over 700 species of bacteria
- Bacteria break down indigestible material, and they also produce some vitamins and other molecules that can be absorbed and used by our bodies.
- Large intestine absorbs water, salt and vitamin
- Bacterial products include gas (mixture of nitrogen&carbon dioxide) with small amounts of hydrogen, methane, and hydrogen sulphide.
- A mucus layer protects the large intestine from attacks from colonic bacterial.
- Bacteria aid in digestion by eating the cellulose in the digested chyme causing waste material to form.
Wednesday, February 2, 2011
Biological Molecules: Water
Technically water is not a biological molecule- it does not contain carbon! However; because of its importance to human physiology it is included here. Water is involved in all the chemical reactions in our bodies. The two main classes of chemical reactions are Synthesis and Hydrolysis. As you read on examples of these reactions will be presented.
Water is a polar molecule. Polar means that each molecule is partially charged. One end (the hydrogens) is positive, and the other (oxygen) end is negative.
Adjacent water molecules will be attracted to each other- the positive end of one molecule to the negative end of another. The connection between the two is called a hydrogen bond. Hydrogen bonds are weak bonds.
Because water is polar it is a good: solvent, temperature regulator, and lubricant.
Water is a polar molecule. Polar means that each molecule is partially charged. One end (the hydrogens) is positive, and the other (oxygen) end is negative.
Adjacent water molecules will be attracted to each other- the positive end of one molecule to the negative end of another. The connection between the two is called a hydrogen bond. Hydrogen bonds are weak bonds.
Because water is polar it is a good: solvent, temperature regulator, and lubricant.
pH Scale by Desiree, Rio, Maede, Mahtab
The pH scale's use is to indicate the acidity or basicity of a solution. The acidity is based on the amount of Hydrogen ions (H+) and the basicity is based on the amount of Hydroxide ions (OH-).


Acids
- taste sour, sharp (ex: Orange)
- Reacts with OH- to form H2O
- Low pH value
- destructive in high amounts
- acidity in the human body: Stomach acid (1), Urine (6), Saliva (6.5)
- Bitter, slippery (ex: Soap)
- Reacts with H+
- Has a high pH value
- destructive in high amounts
- bases in the human body: Stomach antacids (9), Blood (7.4)
From 0 to 7 presents the acids, 7 is neutral (water and tears) while the bases are the number range of 7-14.
Litmus Test
Is used to find out the acidity or the base amount of a solution by means of vegetable dye which changes depending on the amount of Hydrogen or Hydroxide ions.
- Blue to red = acid
- Red to blue = base
A buffer is either a chemical or compound that keeps a constant pH level.
- Example: Our blood has a pH value of 7.4, and hemoglobin and bicarbonate ions acts as a buffer in human blood.
Carbohydrates
Function of carbohydrates:
-function for quick and short term energy storage in all organisms.
-play a structural role in woody plants,bacteria, and animals such as insects.
-carbohydrates on cell surfaces play a role in cell to cell recognition.
Monosacchrides: are the most basic units of biologically important carbohydrates; they are the simplest form of sugar.
If the number of Carbon atoms in a molecule is low (from three to seven carbon atoms) , then the crabohydrate is a simple sugar, or monosacchride.
Glucose: a hexose and blood sugar. Such as fructose found in fruits and galactose a constituent of milk.
Disacchride: made by linking two monosacchrides together.
Maltose: a dissacchride that contains two glucose molecules.
Formation of Maltose:
Sucrose: glucose+fructose---> sucrose, which is commonly known as Table sugar.
By: Rojin,Sarah,Nicole,Fred
Polysaccharides
What is Polysaccharides?
Polysaccharides is the polymer made from sugar monomers; the polysaccharides starch and glycogen are polymers of glucose monomers. Polysaccharides has three kinds; starch, glycogen and cellulose.
Starch
Starch is ready storage forms of glucose in plants. Starch has fewer side branches, or chains of glucose that branch off from the main chain, than does glycogen. Flour, which we acquire by grinding wheat and use for baking, is high in starch, and so are potatoes.

Glycogen
Glycogen is a highly branched polymer of glucose molecules. Glycogen is the storage form of glucose in animals.

Cellulose
Cellulose is found in plant cell walls. In cellulose, the glucose units are joined by different type of linkage than that in starch or glycogen. (Position of the oxygen atoms alternate in linked glucose units.) Cellulose in cell walls permits non-woody plants to stand upright as long as they receive an adequate supply of water.

Polysaccharides is the polymer made from sugar monomers; the polysaccharides starch and glycogen are polymers of glucose monomers. Polysaccharides has three kinds; starch, glycogen and cellulose.
Starch
Starch is ready storage forms of glucose in plants. Starch has fewer side branches, or chains of glucose that branch off from the main chain, than does glycogen. Flour, which we acquire by grinding wheat and use for baking, is high in starch, and so are potatoes.

Glycogen
Glycogen is a highly branched polymer of glucose molecules. Glycogen is the storage form of glucose in animals.

Cellulose
Cellulose is found in plant cell walls. In cellulose, the glucose units are joined by different type of linkage than that in starch or glycogen. (Position of the oxygen atoms alternate in linked glucose units.) Cellulose in cell walls permits non-woody plants to stand upright as long as they receive an adequate supply of water.

Amino Acids and Their Function in Proteins
Proteins
3. Antibodies
Amino Acids
- There are 4 different uses of protein in our body
- Such as "keratin", this makes hair and nails, and collagen.
- Supports ligaments, tendons, and skin
- The plasma membrane of our cells sometimes form channels allowing things to enter and leave cells. These channels are made of protein.
- These are messengers that influence cellular metabolism
3. Antibodies
- These are made out of proteins as well as other body fluids
- These combine with foreign substances, preventing them from destorying cells
- Also prevents from upsetting homeostasis
- Speed up chemical reactions
Amino Acids
- An amino acid is a building block for proteins
- When two Amino Acids join it’s formed into something called a Dipeptide water is also formed

- There are only 20 Amino Acids in the world
- There are 3 parts [Acid + Amino + R group]
- An example of an Amino Acid:

- A peptide bond is associated with oxygen, carbon, nitrogen, and hydrogen
- Oxygen usually has a partial negative charge and Hydrogen a partial Positive
Protein Structure
An enzyme is a protein molecule that functions as an organic catalyst to speed a chemical reaction. The structure of a protein has at least three levels of an organization, and can even have four. Each level can be thought of as a different phase. A polypeptide is a single chain of amino acids. A peptide is a bond that joins two amino acids.
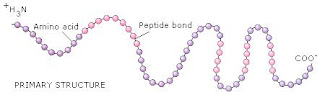



First Level- Primary Structure
A linear sequence of the amino acids joined by peptide bonds (think of the structure like a necklace and the amino acids are the different beads).
Second Level- Secondary Structure
The second structure comes about when the polypeptide takes on a certain orientation in space, the structure is an alpha helix. Hydrogen bonding between peptide bongs holds the shape in place.


Third Level- Tertiary Structure
The structure is now finally a three-dimensional shape in this structure. The helix folds into a
characteristic globular shape due in part to covalent bonding between 'R' groups. ('R' groups are alanine, valine, cysteine, and phenylalnine.) The shape is maintained by various types of bonding; covalent, ionic, and hydrogen.
Fourth Level- Quaternary Structure
This level occurs when two or more polypeptides join to form a single protein.
Proteins, which have levels of organization, are important in the structure and the function of cells. Some proteins are enzymes, which speed chemical reactions.
Abby
Kristen
Cooper
Erik
Nucleic Acids
Nucleic Acids
Here are two types of nucleic acids; DNA and RNA. DNA is deoxyribonucleic acid, and RNA is ribonucleic acid. DNA stores genetic information in the cell. It then replicates and transmits this info when a cell reproduces, DNA codes for the order in which amino acids are to be joined to form a protein. RNA is an intermediary that conveys DNA’s instructions regarding the amino acid sequence in a protein.
 |
| Dna |
-There are four different types of base in DNA: A=adenine, T=thymine, G=guanine, and C= cytosine
- The base can have two rings (adenine or guanine) or one ring (thymine or cytosine). These structures are called bases because their presence raises the pH of a solution.
-DNA is double stranded, with the two strands twisted about each other in the form of a double helix.
-The two strands are held together by hydrogen bonds between the base,
-T always pairs with A, and G with C.
-the uprights of the ladder are made entirely of phosphate and sugar molecules, and the rungs are made only of complementary paired bases.
Dna structure compared to Rna structure
Dna Rna
Sugar | Deoxyribose | Ribose |
Bases | Adenine, guanine, thymine, cytosine | Adenine, guanine, uracil, cytosine |
strands | Double stranded with base pairings | Single stranded |
helix | yes | no |
 |
| Rna |
-An energy carrier in cells
- high-energy molecule because the last two phosphate bonds are unstable and easily broken.
-composed of adenosine and three phosphate groups
-after energy is release by ATP breakdown it is used by the cell to synthesize macromolecules (carbohydrates and proteins)
-ATP undergoes hydrolysis and breakdown to produce ADP + P
By: Katie, Monica, Anne, Anna
Lipids!
A lipid is an organic compound that is insoluble in water; notably fats, oils and steroids.
- Their low solubility in water is due to an absence of polar groups.
- They contain little oxygen and consist mostly of carbon and hydrogen atoms.
- The most familiar lipids are those found in fats and oils.
- Fats are usually from an animal origin(lard and butter) that are solid at room temperature.
- Oils which are usually from a plant origin(corn oil and soybean oil) are liquid at room temperature.
- Fat has many functions in the body:it is used for long term energy storage, it insulates against heat loss, and it forms protective cushion around major organs.
- Neutral fat is sometimes used because the molecule is non-polar.
Fatty acid- A molecule that contains a hydrocarbon chain and ends with an acid group. There are two types of fatty acids; saturated and unsaturated fatty acids.
Difference between unsaturated fatty acids and unsaturated fatty acids?

Difference between unsaturated fatty acids and unsaturated fatty acids?

Saturated fatty acid- A molecule that lacks double bonds between the carbons and the hydrocarbon chain.The chain bears the maximum number of hydrogen's.
Unsaturated fatty acid- A fatty acid molecule that has one or more double bonds between the atoms of its carbon chain.
Unsaturated fatty acid- A fatty acid molecule that has one or more double bonds between the atoms of its carbon chain.

A phospholipid is a molecule that forms the bilayer of the cells of the cells membranes; has a polar, hydrophilic head bonded to two nonpolar, hydrophobic tails

Cholesterol and testosterone are the two steroid molecules; they act as chemical messengers.


Subscribe to:
Posts (Atom)







